How to Draw 100 Plane
Lattice Planes and Miller Indices (all content)
Note: DoITPoMS Education and Learning Packages are intended to be used interactively at a computer! This print-friendly version of the TLP is provided for convenience, just does non display all the content of the TLP. For example, any video clips and answers to questions are missing. The formatting (folio breaks, etc) of the printed version is unpredictable and highly dependent on your browser.
Contents
Chief pages
Additional pages
Aims
On completion of this TLP you lot should:
- Empathize the concept of a lattice plane;
- Be able to make up one's mind the Miller indices of a airplane from its intercepts with the edges of the unit prison cell;
- Be able to visualise and describe a plane when given its Miller indices;
- Be enlightened of how knowledge of lattice planes and their Miller indices can aid to understand other concepts in materials scientific discipline.
Before you start
You should understand the concepts of a lattice, unit jail cell, crystal axes, vrystal system and the variations, primitive, FCC, BCC which brand upwardly the Bravais lattice.
You might also like to look at the TLP on Atomic Scale Structure of Materials.
You lot should empathize the concepts of vectors and planes in mathematics.
Introduction
Miller Indices are a method of describing the orientation of a plane or set of planes inside a lattice in relation to the unit of measurement cell. They were adult by William Hallowes Miller.
These indices are useful in understanding many phenomena in materials science, such as explaining the shapes of single crystals, the class of some materials' microstructure, the estimation of Ten-ray diffraction patterns, and the movement of a dislocation, which may determine the mechanical properties of the textile.
How to index a lattice plane
The next three animations take you lot through the basics of how to index a plane. Click "Start" to begin each animation, and then navigate through the pages using the buttons at the bottom right.
Parallel lattice planes
This animation explains the relationships between parallel planes and their indices. Click "Start" to begin and use the buttons at the bottom right to navigate through the pages.
Lattice planes tin be represented past showing the trace of the planes on the faces of ane or more unit of measurement cells. The diagram shows the trace of the (23) planes on a cubic unit cell.
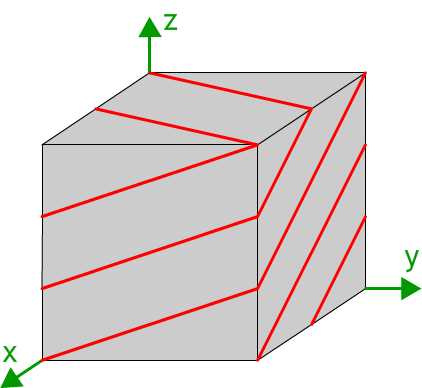
How to draw a lattice plane
Bracket Conventions
In crystallography there are conventions as to how the indices of planes and directions are written. When referring to a specific plane, "round" brackets are used:
(hkl)
When referring to a set of planes related by symmetry, then "curly" brackets are used:
{hkl}
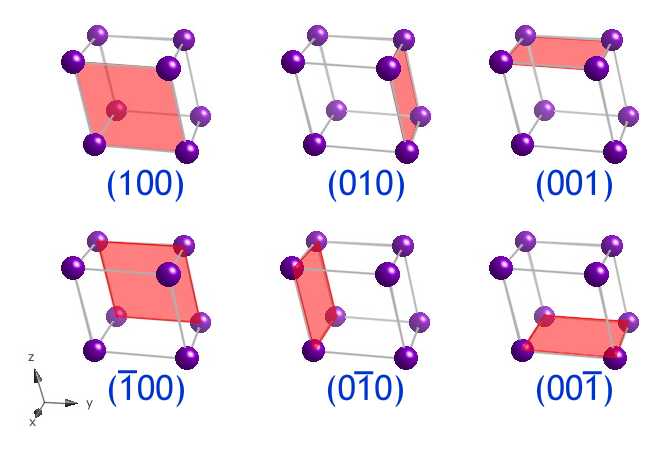
These might exist the (100) type planes in a cubic system, which are (100), (010), (001), (00),(00) and (00) . These planes all "look" the same and are related to each other by the symmetry elements present in a cube, hence their different indices depend only on the way the unit cell axes are divers. That is why it useful to consider the equivalent (010) set up of planes.
Directions in the crystal can be labelled in a similar way. These are finer vectors written in terms of multiples of the lattice vectors a, b, and c. They are written with "square" brackets:
[UVW]
A number of crystallographic directions can also exist symmetrically equivalent, in which instance a set of directions are written with "triangular" brackets:
<UVW>
Vectors and Planes
It may seem, after considering cubic systems, that whatever lattice plane (hkl) has a normal direction [hkl]. This is not always the instance, as directions in a crystal are written in terms of the lattice vectors, which are non necessarily orthogonal, or of the same magnitude. A simple instance is the example of in the (100) plane of a hexagonal system, where the direction [100] is actually at 120° (or 60° ) to the plane. The normal to the (100) airplane in this case is [210]
VR rotating image
Weiss Zone Law
The Weiss zone constabulary states that:
If the direction [UVW] lies in the aeroplane (hkl), then:
hU +kV +lW = 0
In a cubic system this is exactly analogous to taking the scalar product of the direction and the aeroplane normal, then that if they are perpendicular, the angle between them, θ, is xc° , and so cosθ = 0, and the management lies in the plane. Indeed, in a cubic organisation, the scalar production can be used to determine the angle betwixt a direction and a aeroplane.
Notwithstanding, the Weiss zone constabulary is more general, and can be shown to work for all crystal systems, to decide if a direction lies in a plane.
From the Weiss zone police the following rule tin can be derived:
The direction, [UVW], of the intersection of (h 1 chiliad one l 1) and (h 2 k ii l 2) is given past:
U =k i l 2 −k 2 l 1
5 =fifty 1 h 2 −50 2 h 1
W =h 1 k two −h two one thousand ane
Equally it is derived from the Weiss zone police force, this relation applies to all crystal systems, including those that are non orthogonal.
Examples of lattice planes
The (100), (010), (001), (00), (00) and (00) planes form the faces of the unit cell. Here, they are shown as the faces of a triclinic (a ≠ b ≠ c, α ≠β ≠γ) unit cell . Although in this prototype, the (100) and (00) planes are shown as the front and back of the unit cell, both indices refer to the same family of planes, as explained in the animation Parallel lattice planes. It should be noted that these 6 planes are not all symmetrically related, as they are in the cubic system.
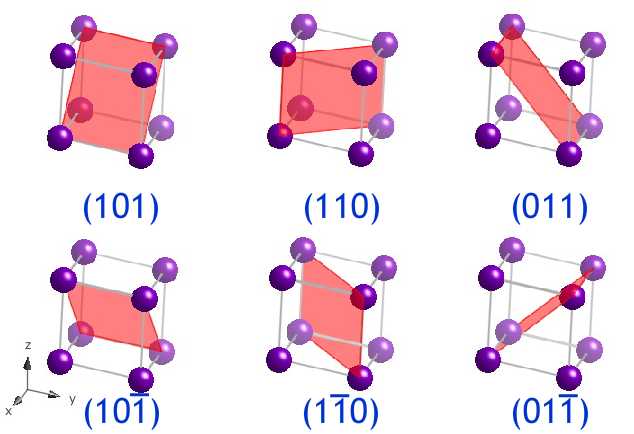
The (101), (110), (011), (10), (10) and (01) planes grade the sections through the diagonals of the unit prison cell, forth with those planes whose indices are the negative of these. In the image the planes are shown in a different triclinic unit of measurement cell.
The (111) type planes in a face centred cubic lattice are the close packed planes.
Click and elevate on the image below to see how a close packed (111) aeroplane intersects the fcc unit of measurement cell.
VR rotating paradigm
Depict your ain lattice planes
This simulation generates images of lattice planes. To meet a plane, enter a set of Miller indices (each alphabetize between 6 and −6), the numbers separated by a semi-colon, then click "view" or press enter.
Applied Uses
An agreement of lattice planes is required to explain the form of many microstructural features of many materials. The faces of single crystals form on certain lattice planes, typically those with low indices.
In a similar mode, the grade of the microstructure in a polycrystalline textile is strongly dependent on lattice planes. When a new stage of material forms, the surfaces tend to be aligned on low alphabetize planes, every bit with single crystals. When a new solid phase is formed in another solid, the interfaces occur on along the most energetically favourable planes, where the two lattices are well-nigh coherent. This leads to plate-similar precipitates forming, at specific angles to each other.

Section through an Fe-Ni meteorite showing plates at lx° to each other
![]() DoITPoMS standard terms of use
DoITPoMS standard terms of use
One method of plastic deformation is by dislocation skid. Understanding lattice planes, and directions is essential to explain why dislocations move, combine and tangle in the observed style. More than data can exist obtained in the TLP - 'Skid in Single Crystals'
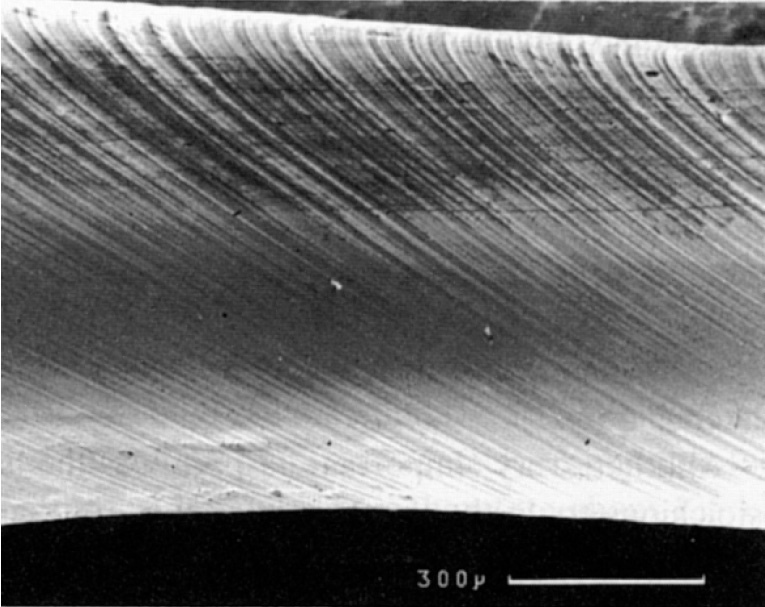
A scanning electron micrograph of a single crystal of cadmium
deforming by dislocation slip on 100 planes, forming steps
on the surface
![]() DoITPoMS standard terms of employ
DoITPoMS standard terms of employ
Twinning is where a part of the crystal is "flipped" to form a mirror epitome of the rest of the crystal, reflected in a item lattice plane. This tin can either occur in annealing, or as a mechanism of plastic deformation.

Annealing twins in brass (DoITPoMS micrograph library)
X-ray diffraction is a method of determining the crystal construction of a material. By interpreting the diffraction patterns equally reflections from lattice planes in the material, the construction can be adamant. More information can be obtained in the TLP - 'X-ray diffraction '
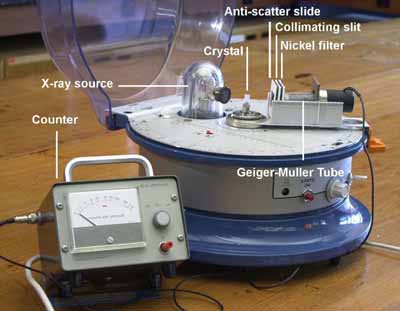
Apparatus for carrying out unmarried crystal X-ray diffraction.
Worked examples
Example A
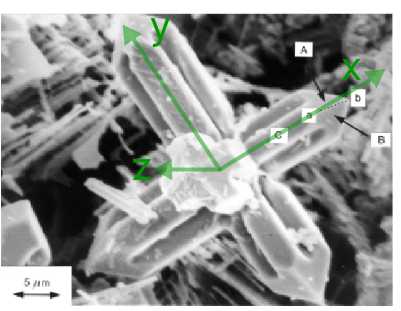
The figure below is a scanning electron micrograph of a niobium carbide dendrite in a Fe-34wt%Cr-5wt%Nb-four.5wt%C alloy. Niobium carbide has a face centred cubic lattice. The specimen has been deep-etched to remove the surrounding matrix chemically and reveal the dendrite. The dendrite has three sets of "artillery" which are orthogonal to ane another (one ready pointing out of the plane of the image, the other two sets, to a skilful approximation, lying in the aeroplane of the paradigm), and each arm has a pyramidal shape at its finish. It is known that the crystallographic directions along the dendrite arms represent to the < 100 > lattice directions, and that the direction ab labelled on the micrograph is [10] .

sourced from Dendritic Solidification
1) If point c (not shown) lies on the axis of this dendrite arm, what is the direction cb ? Alphabetize face C , marked on the micrograph.
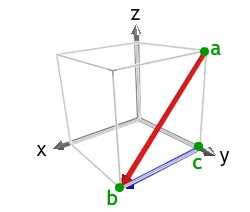
The diagram shows the [x] direction in ruddy. The [100] management is a < 100 > type direction that forms the observed acute angle with ab, and tin exist used as cb. Of the < 100 > type directions, we could as well accept used [00] .
Using a right handed gear up of axes, we then have z-centrality pointing out of the plane of the image, the ten-axis pointing forth the direction cb, and the y-axis pointing towards the top left of the epitome.
Confront C must contain the management cb, and its normal must point out of the airplane of the image. Therefore face C is a (001) airplane.
2) The four faces which lie at the end of each dendrite arm accept normals which all make the same bending with the direction of the arm. Observing that faces A and B marked on the micrograph both contain the direction ab , and noting the full general directions along which the normals to these faces point, alphabetize faces A and B .
Both faces A and B have normals pointing in the positive x and z directions, i.due east. positive h and l indices. Face A has a positive k index, and face B has a negative k alphabetize.
The morphology of the ends of the arms is that of one-half an octahedron, suggesting that the faces are (111) type planes. This would make face A, in green, a (111) plane, and confront B, in blue, a (one1) aeroplane. As required, they both contain the [10] direction, in red.
Example B
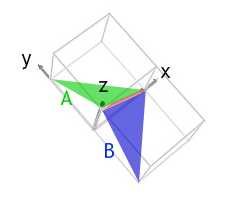
i) Work out the common direction between the (111) and (001) in a triclinic unit cell.
The relation derived from the Weiss zone police in the section Vectors and planes states that:
The direction, [UVW], of the intersection of (h 1 k 1 50 1) and (h 2 1000 two l 2) is given by:
U =m 1 l 2 −k 2 l 1
V =50 1 h 2 −l 2 h i
W =h 1 k two −h 2 k 1
We can use this relation equally information technology applies to all crystal systems, including the triclinic system that we are considering.
We have h 1 = ane, k one = ane, l 1 = ane
and h two = 0, k 2 = 0, l ii = ane
Therefore
U = (1 × 1) - (0 × one) = 1
V = (1 × 0) - (1 × 1) = −1
W = (1 × 0) - (0 × 1) = 0.
So the common direction is:
[one0] .
This is shown in the image below:
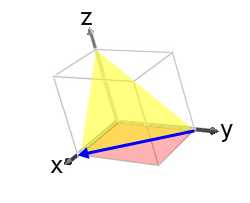
If we had divers the (001) plane as (h 1 k 1 l 1) and the (110) plane every bit (h 2 grand two 50 2) then the resulting direction would have been, [x] i.due east. anti-parallel to [10] .
ii) Use the Weiss zone constabulary to show that the direction [i0] lies in the (111) airplane.
Nosotros have U =1, V =−i, West =0,
and h = ane, k = one, 50 = one.
hU +kV +lW = (1 × 1) + (one × −1) + (1 × 0) = 0
Therefore the direction [ane0] lies in the plane (111).
Summary
Miller Indices are the convention used to label lattice planes. This mathematical clarification allows us to ascertain accurately, planes within a crystal, and quantitatively analyse many problems in materials science.
Questions
Game: Identify the planes
Quick questions
Y'all should be able to answer these questions without likewise much difficulty after studying this TLP. If not, then y'all should go through it again!
-
Which one of the following statements about the (4) and (21) planes is false?
-
Does the [12] direction lie in the (30) airplane?
-
When writing the alphabetize for a fix of symmetrically related planes, which type of brackets should be used?
-
Which of the <110> blazon directions lie in the (112) plane?
-
What is the common direction between the (1 ) and (33) planes?
-
Which set of planes in a cubic-close-packed structure (such as copper) is close packed?
Open-concluded questions
The post-obit questions are non provided with answers, just intended to provide food for thought and points for further give-and-take with other students and teachers.
-
Practice sketching some lattice planes. Make sure yous can draw the {100}, {110} and {111} type planes in a cubic organization.
-
Draw the trace of all the (21) planes intersecting a cake 2 × 2 × two cake of orthorhombic (a ≠ b ≠ c, α = β = γ = 90°) unit cells.
-
Sketch the arrangement of the lattice points on a {111} blazon plane in a face centred cubic lattice. Do the same for a {110} type plane in a torso centred cubic lattice. Compare your drawings. Why practise you think the {110} type planes are ofttimes described equally the "most close packed" planes in bcc?
Going further
Books
[i] D. McKie and C. McKie, Crystalline Solids , Thomas Nelson and Sons, 1974.
A very comprehensive crystallography text.
[2] C. Hammond, The Basics of Crystallography and Diffraction , Oxford, 2001.
Chapter 5 covers lattice planes and directions. The rest of the book gives an introduction to crystallography and diffraction in general.
[3] B.D. Cullity, Elements of Ten-Ray Diffraction , Prentice Hall, 2003.
Covers X-Ray diffraction in particular. Chapter 2 covers the crystallography required for this.
[4] C. Kittel, Introduction to Solid State Physics, John Wiley and Sons, 2004.
Chapter ane covers crystallography. The book then goes on to cover a broad range of more advanced solid country science.
Cómo indexar un plano de una red
Las siguientes tres animaciones muestran los fundamentos básicos para calcular los parámetros del crimson. Haz click en "Inicio" para que comience cada animación, y luego navega a través de las páginas usando los botones situados en la parte junior derecha.
如何标注一个晶格面
以下的三个动画 课程将让你了解关于标注晶格面的基本知识。点击'开始'来开始每个动画课程,然后用右下角的按钮来进入下一页。
Как обозначать плоскость кристаллической решётки
Следующие три анимации покажут основы того, как обозначать плоскость. Нажмите кнопку "Пуск", чтобы запустить каждую из анимаций, а затем управляйте анимацией с помощью кнопок, расположенных в правом нижнем углу.
Bookish consultant: Noel Rutter (University of Cambridge)
Content development: Peter Marchment
Photography and video: Brian Barber and Carol Best
Web evolution: David Beck and Lianne Sallows
Translation: Jing Qiu, Kansong Chen, Ana Tabalan-Bailey, Marta Sanchez, Juan Vilatela
DoITPoMS is funded past the UK Eye for Materials Educational activity and the Department of Materials Science and Metallurgy, Academy of Cambridge
Additional support for the evolution of this TLP came from the Worshipful Company of Armourers and Brasiers'
Source: https://www.doitpoms.ac.uk/tlplib/miller_indices/printall.php
Post a Comment for "How to Draw 100 Plane"